Quality Systems & Processes
Quality Systems
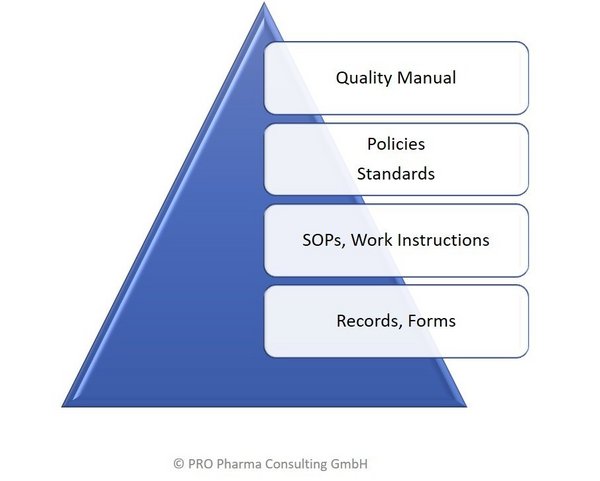
The Quality Management System, QMS, of a company defines the structure of the documentation system, the document hierarchy in relation to Policies, Standards, SOPs and Work Practices. It is key to any GxP- or Medical Device regulated industry to create and maintain a consistent, scaleable, and harmonized QMS.
PRO Pharma Consulting can support you in creating, deplyoing and/or improving a Quality Management System. The QMS has to be aligned with the Vision & Mission of a company and is an import value driver and ultimately helping to protect our Patients.
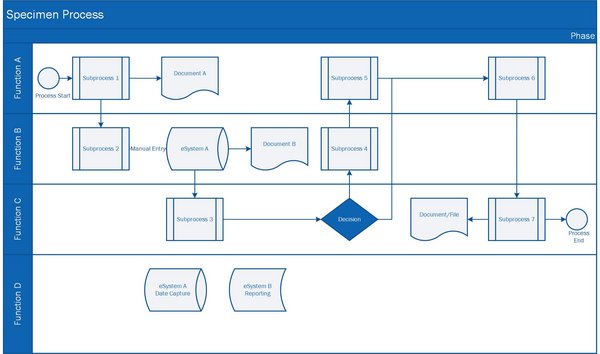
Processes
Understanding, defining, and optimizing Processes are part of a agile, solid, and effective Quality Management System. "PRO" in the name of PRO Pharma Consulting is derived from Processes. Using modern principles and tools (MS Visio), we can help you to design and visualize processes. Cross-functional workshops, including all involved functions & stakeholders will serve as a basis to develop a process. The workshop itself already has its value to reach a common understanding of a process and the the respective responsibilities.